Drug repurposing, also called drug repositioning, reprofiling or retasking, is the process of identifying a new use for an existing medicine/active substance outside the scope of the original indication(s). It includes new therapeutic uses for existing medicines, different formulations of the same medicine, and/or creating new combinations of medicines or medicines with medical devices[1].
Drug repurposing has recently attracted much attention as it promises faster timelines, lower costs and less risk compared to new drug development. In this blog the importance, advantages, and challenges of drug repurposing including the overall regulatory framework that must be considered are described.
The Need for Innovation
In recent years, there has been an increased awareness of the need to improve the efficiency and effectiveness of drug development and that access to new medicines should be accelerated. In this light, repurposing of drugs has gained growing interest as it could facilitate earlier access to medicines for unmet medical needs (i.e. medicines intended for treating, preventing, or diagnosing seriously debilitating or life-threatening diseases such as rare diseases) or pediatric medicines.
As generally known development of traditional (de novo) medicines is time-consuming and requires large financial investments, and yet the chance of success is not self-evident. From drug development until marketing authorisation, the approval from a health authority to market a medicine in a country can easily take more than 10-15 years including drug discovery, non-clinical trials (in vitro and in vivo), clinical trials (phase I (safety), phase II (dose-finding/efficacy), phase III (safety/efficacy)) and drug product registration.
Drug Repurposing by Accident
New indications for existing drugs are discovered frequently by accident, for example during real-world market use or during clinical trials. The most well-known repurposed drug is sildenafil, known as Viagra®. Sildenafil was originally developed for the treatment of hypertension (high blood pressure) and angina pectoris (chest pain due to heart disease). During clinical trials, an unexpected side effect was observed: the induction of erections.[2]
Another example of a repurposed drug is semaglutide (Ozempic®), originally indicated for type 2 diabetes. During pre-approval studies, researchers noticed a remarkable side effect: weight loss. The drug was repurposed for obesity (with a slightly higher dose than for type 2 diabetes) and approved under the name Wegovy.[3]
Although these examples of drug repurposing are valuable, it would be beneficial if repurposing would be done intentionally and in a more structured and efficient way.
Advantages of Drug Repurposing
By using existing medicines, some of the early stages of development, such as the pre-clinical phase of compound discovery, refinement, safety testing, and phase I safety studies in humans, can be omitted as the safety profiles of these drugs are already well-known. This definitively will lead to shorter development timelines and a reduction in development costs.
A schematic overview of the difference between de novo drug development and drug repurposing timelines is provided in Figure 1.
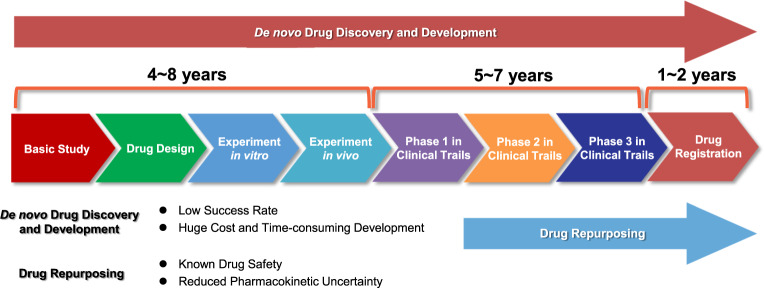
Figure 1. Difference between de novo drug development and drug repurposing timelines (from Zhang et al.[4] used under Creative Commons CC-BY license.)
Challenges in the Repurposing Journey
To what extent a drug can be repurposed highly depends on the data that is available for that drug. The drug to be repurposed can be divided into 3 groups:
-
- A medicine that has never been approved by regulators,
- A medicine that is still under intellectual property (IP) or regulatory data protection,
- A well-known medicine that is not IP-protected.
It is difficult to obtain data for unauthorised drugs and data for medicines under IP and regulatory protections, and the data available might not be usable to obtain a marketing authorisation without approval from the drug/license holder. Therefore, medicines without any protection are ideally suited for drug repurposing. However, identifying the right candidates for repurposing requires a deep understanding of the underlying biological mechanisms of diseases and the pharmacological properties of existing drugs. Additionally, there may be legal and financial considerations, such as obtaining new patents for known drugs, which can be complex.
The extent of potential bypassing certain development stages depends on the similarity between the underlying biological mechanisms of the new indication and the original one, as well as the necessity for drug reformulation. The further away from the original mechanism and/or formulation, the more stages of development need to be performed.
Technological Advances and Data Mining
New technologies in the field of artificial intelligence (AI), machine learning, and data mining techniques and the availability of big health datasets have significantly expanded the field of drug repurposing. Complex computational algorithms can process large amounts of real-world data. It can analyse complex biological datasets which can lead to the discovery of potential drug and/or disease interactions, leading to new uses for existing medications.
An example of the process of drug repurposing in COVID-19 using AI is shown in Figure 2. The data related to drugs, diseases, proteins, and genes, etc. are extracted from different data sources (databases) followed by the use of different repurposing methods. Repurposing methods are used to determine the potential drugs, which are then validated in in vitro (2D or 3D) cell culture and in vivo animal models. Finally, the shortlisted drugs are forwarded to clinical trials and successful drugs are repurposed[5].
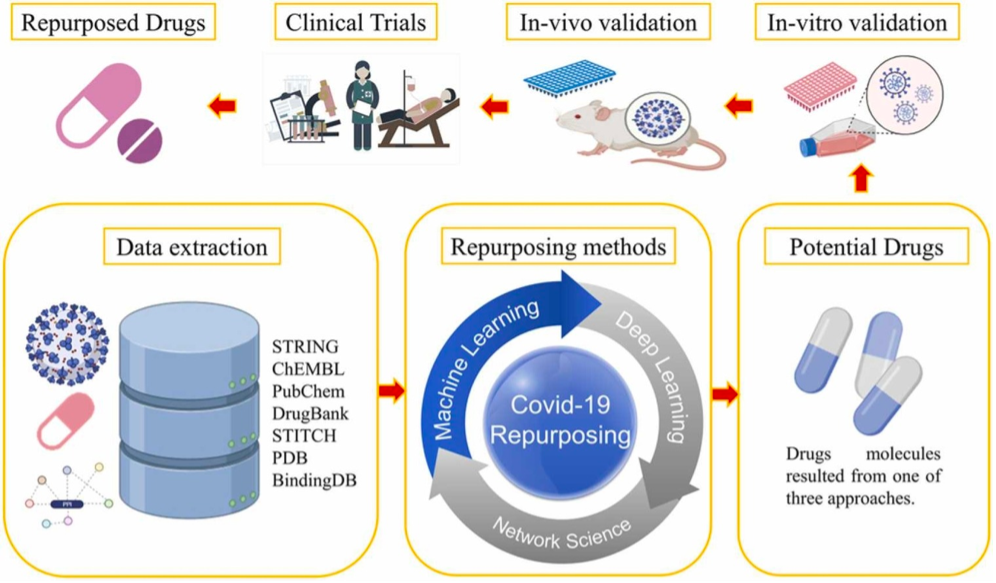
Figure 2. Drug repurposing in COVID-19 using AI (from Ahmed et al.[5], used under Creative Commons CC-BY license.)
REPO4EU (Precision Drug REPurpOsing For EUrope and the World)
3D-PharmXchange is taking part in the innovative drug repurposing consortium REPO4EU. REPO4EU is a Horizon Europe project launched in 2022 as an ambitious joint initiative that aims to create a connected European Platform for Drug Repurposing. REPO4EU’s ultimate goal is to build and grow an industry-level European online platform for validated precision drug repurposing with a global reach. Instead of repurposing medicines to treat the symptoms of a disease, it will focus on the network of mechanism behind it. Combining therapies will be needed as it becomes more and more clear that hardly any disease can be neither explained nor cured via one single target. Mechanism-based drug repurposing will start by using advanced bioinformatics and AI techniques, as mentioned above, on real-world big data. This will lead to more precise and effective medicines.
When using these kinds of innovative techniques it is advised to discuss the use of it with the regulatory authorities in an early stage of drug development to make sure it fits in the regulatory requirements.
The Regulatory Framework
Currently, no specific regulatory guideline for drug repurposing exists. The new indication for a repurposed drug can be registered following the currently applicable European legal guidelines:
- variation or line extension via Reg (EC) No 1234/2008 for an already registered drug. The indication will be added to the already existing marketing authorisation. This addition should be done by the current Marketing Authorisation Holder (MAH). However, adding a new indication to an already registered product will lack additional incentives (maximum of 1 year market and/or data protection). For this reason, pharmaceutical companies have less interest in repurposing their well-known drugs.
- submission of a new full or abridged marketing authorisation via Directive 2001/83/EC. A full marketing authorisation will give the developer 8 years of data protection and 10 years of market exclusivity but also a full package of non-clinical and clinical data needs to be provided. When (other) drug developers will authorise the repurposed drug via the abridge route (hybrid or biosimilar) no incentives will be received at all. Only when a drug is repurposed for orphan diseases or paediatric use the incentives could be interesting (8-12 years of data and/or market protection).
Regulatory authorities also see the advantage of drug repurposing. The European Commission has drafted an update of Directive 2001/83/EC (Union code relating to medicinal products for human use) that now includes an article for data protection of repurposed medicinal products for medicines that are registered via the generic, hybrid or (hybrid)-biosimilar route. This should stimulate other drug developers to repurpose drugs.
Scientific Advice
The development of an approved drug for a new indication is attractive and more efficient than developing a new drug because there is already a great volume of safety and efficacy data available on that drug. Nevertheless, drug repurposing must follow a similar regulatory approval path as for traditional drug development, meaning that the final goal of the development program is to bring a safe and efficacious repurposed drug to patients. It is advisable to seek scientific advice from a regulatory authority in an early stage of drug repurposing. The benefits of seeking scientific advice are early regulatory input, knowing the regulatory perspective, trial optimisation and risk mitigation. It will give valuable input if the shortened development program is acceptable for marketing authorisation of the repurposed drug at the end.
3D-PharmXchange in Drug Repurposing
In our pursuit of repurposed drugs, we 3D-PharmXchange are committed to innovation and comprehensive development from inception to market for our clients. Our expertise encompasses a spectrum of crucial development activities, including reformulation, manufacturing, nonclinical toxicology strategy and execution, clinical strategy and study execution, pharmacokinetics (PK) and pharmacodynamics (PD) analysis, and regulatory affairs strategy, including orphan drug designation, and interaction with FDA, EMA and national authorities and patent strategy. Through our dedicated efforts, we have facilitated the transformation of existing drugs into potent solutions for new therapeutic indications.
For instance, we have managed the reformulation of edaravone into an oral formulation, originally developed for stroke, now repurposed for neurodegenerative diseases. Additionally, we've successfully repurposed a medicine initially intended for thrombocythaemia, and now for soft tissue sarcomas. Another notable example is the reformulation of midazolam as a nasal spray, unlocking a new indication for the treatment of prolonged, acute, convulsive seizures. Moreover, our expertise extends to the repurposing of existing medicines for conditions like NF1, highlighting our dedication to addressing unmet medical needs through innovative approaches. These are just a few examples of 3D-PharmXchange experience, and we combine science, development experience, and strategic insights to unlock the full potential of repurposed drugs, driving innovation in healthcare and advancing patient outcomes.
References
-
- Asker-Hagelberg, C., Boran, T., Bouygues, C., Eskola, S. M., Helmle, L., Hernández, C., Houýez, F., Lee, H., Lingri, D., Louette, L., Meheus, L., Penninckx, W., Stepniewska, B. Repurposing of Medicines in the EU: Launch of a Pilot Framework. Frontiers in Medicine 2022;8:817663. https://doi.org/10.3389/fmed.2021.817663
- https://www.drugs.com/article/viagra-little-blue-pill.html
- https://www.health.harvard.edu/blog/what-happens-when-a-drug-goes-viral-202302212892
- Zhang, Z., Zhou, L., Xie, N. Nice E.C, Zhang T. Overcoming cancer therapeutic bottleneck by drug repurposing. Signal Transduction & Target Therapy 2020;5:113. https://doi.org/10.1038/s41392-020-00213-8
- Ahmed, F., Soomro, A. M., Salih, A. R. C., Samantasinghar, A., Asif, A., Kang, I. S., Choi, K. H. A comprehensive review of artificial intelligence and network based approaches to drug repurposing in Covid-19. Biomedicine & Pharmacotherapy 2022 (9);153:113350. https://doi.org/10.1016/j.biopha.2022.113350

